In What Stage Is a Cell That Will Not Divide Again


Onion (Allium) cells in different phases of the prison cell cycle. Growth in an 'organism' is carefully controlled by regulating the prison cell cycle.
Cell cycle in Deinococcus radiodurans
The jail cell cycle, or cell-sectionalization bike, is the series of events that take identify in a cell that crusade information technology to separate into two daughter cells. These events include the duplication of its DNA (DNA replication) and some of its organelles, and afterward the partitioning of its cytoplasm and other components into ii daughter cells in a process called prison cell division.
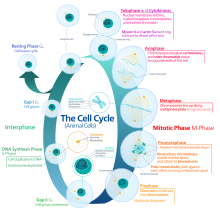
In cells with nuclei (eukaryotes, i.e., animal, plant, fungal, and protist cells), the cell cycle is divided into two main stages: interphase and the mitotic (M) phase (including mitosis and cytokinesis). During interphase, the cell grows, accumulating nutrients needed for mitosis, and replicates its Dna and some of its organelles. During the mitotic phase, the replicated chromosomes, organelles, and cytoplasm dissever into two new girl cells. To ensure the proper replication of cellular components and partition, in that location are control mechanisms known every bit prison cell bike checkpoints after each of the key steps of the bike that determine if the cell tin progress to the next stage.
In cells without nuclei (prokaryotes, i.due east., bacteria and archaea), the cell cycle is divided into the B, C, and D periods. The B period extends from the terminate of jail cell division to the beginning of Dna replication. Dna replication occurs during the C period. The D period refers to the stage between the end of DNA replication and the splitting of the bacterial cell into two daughter cells.[1]
The jail cell-segmentation wheel is a vital procedure past which a single-celled fertilized egg develops into a mature organism, equally well as the process by which hair, skin, blood cells, and some internal organs are regenerated and healed (with possible exception of nerves; run into nervus impairment). After cell division, each of the girl cells begin the interphase of a new cell bike. Although the diverse stages of interphase are not commonly morphologically distinguishable, each phase of the prison cell wheel has a distinct set of specialized biochemical processes that fix the prison cell for initiation of the prison cell division.
Phases [edit]
The eukaryotic prison cell cycle consists of four distinct phases: One thousand1 stage, S phase (synthesis), 1000two phase (collectively known every bit interphase) and Thousand phase (mitosis and cytokinesis). M stage is itself composed of ii tightly coupled processes: mitosis, in which the cell'southward nucleus divides, and cytokinesis, in which the prison cell'southward cytoplasm divides forming two daughter cells. Activation of each stage is dependent on the proper progression and completion of the previous one. Cells that have temporarily or reversibly stopped dividing are said to have entered a country of quiescence called K0 phase.
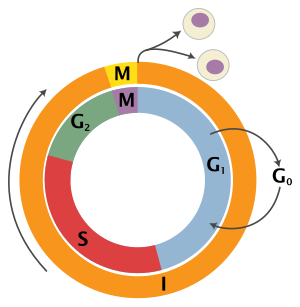
| State | Stage | Abridgement | Description |
|---|---|---|---|
| Resting | Gap 0 | Yard0 | A phase where the prison cell has left the cycle and has stopped dividing. |
| Interphase | Gap 1 | Grand1 | Jail cell growth. The Gone checkpoint ensures that everything is ready for Dna synthesis. |
| Synthesis | South | DNA replication. | |
| Gap 2 | Chiliad2 | Growth and preparation for mitosis. The G2 checkpoint ensures that everything is ready to enter the M (mitosis) stage and divide. | |
| Cell division | Mitosis | 1000 | Cell sectionalisation occurs. The Metaphase Checkpoint ensures that the cell is fix to complete cell division. |
Subsequently jail cell partitioning, each of the daughter cells brainstorm the interphase of a new cycle. Although the various stages of interphase are not usually morphologically distinguishable, each phase of the prison cell bike has a singled-out set of specialized biochemical processes that set up the jail cell for initiation of cell division.
G0 phase (quiescence) [edit]
G0 is a resting phase where the cell has left the cycle and has stopped dividing. The cell cycle starts with this phase. Non-proliferative (non-dividing) cells in multicellular eukaryotes generally enter the quiescent G0 state from Gane and may remain quiescent for long periods of time, perhaps indefinitely (as is frequently the instance for neurons). This is very common for cells that are fully differentiated. Some cells enter the M0 phase semi-permanently and are considered post-mitotic, e.g., some liver, kidney, and stomach cells. Many cells do not enter G0 and continue to divide throughout an organism's life, eastward.g., epithelial cells.
The word "postal service-mitotic" is sometimes used to refer to both quiescent and senescent cells. Cellular senescence occurs in response to Dna damage and external stress and usually constitutes an abort in Grandone. Cellular senescence may make a jail cell's progeny nonviable; information technology is oftentimes a biochemical culling to the self-destruction of such a damaged cell by apoptosis.
Interphase [edit]
Interphase represent the phase between 2 successive M phases. Interphase is a series of changes that takes place in a newly formed cell and its nucleus before it becomes capable of division again. It is also called preparatory stage or intermitosis. Typically interphase lasts for at to the lowest degree 91% of the full time required for the cell wheel.
Interphase gain in 3 stages, One thousandone, S, and Grand2, followed past the bicycle of mitosis and cytokinesis. The cell'south nuclear DNA contents are duplicated during S phase.
K1 phase (Commencement growth phase or Post mitotic gap stage) [edit]
The first phase inside interphase, from the finish of the previous M stage until the outset of Dna synthesis, is chosen Grandone (G indicating gap). It is also called the growth phase. During this phase, the biosynthetic activities of the prison cell, which are considerably slowed downwardly during M phase, resume at a high rate. The duration of Ki is highly variable, even among dissimilar cells of the same species.[3] In this phase, the cell increases its supply of proteins, increases the number of organelles (such as mitochondria, ribosomes), and grows in size. In G1 stage, a jail cell has three options.
- To keep cell wheel and enter S phase
- Finish cell cycle and enter Yard0 stage for undergoing differentiation.
- Go arrested in Gone phase hence it may enter G0 phase or re-enter cell cycle.
The deciding betoken is called check point (Restriction indicate). This check point is chosen the restriction point or Showtime and is regulated by K1/Southward cyclins, which cause transition from Gi to Due south stage. Passage through the M1 check signal commits the prison cell to division.
South stage (Deoxyribonucleic acid replication) [edit]
The ensuing S stage starts when DNA synthesis commences; when it is consummate, all of the chromosomes have been replicated, i.eastward., each chromosome consists of two sis chromatids. Thus, during this phase, the amount of Deoxyribonucleic acid in the cell has doubled, though the ploidy and number of chromosomes are unchanged. Rates of RNA transcription and protein synthesis are very low during this phase. An exception to this is histone production, nigh of which occurs during the South phase.[iv] [5] [6]
G2 stage (growth) [edit]
One thousandtwo stage occurs after DNA replication and is a period of protein synthesis and rapid prison cell growth to prepare the cell for mitosis. During this phase microtubules begin to reorganize to form a spindle (preprophase). Before proceeding to mitotic stage, cells must exist checked at the Thousandtwo checkpoint for any Dna impairment within the chromosomes. The Thousand2 checkpoint is mainly regulated past the tumor protein p53. If the Deoxyribonucleic acid is damaged, p53 will either repair the Deoxyribonucleic acid or trigger the apoptosis of the prison cell. If p53 is dysfunctional or mutated, cells with damaged DNA may continue through the prison cell cycle, leading to the development of cancer.
Mitotic phase (chromosome separation) [edit]
The relatively cursory One thousand phase consists of nuclear partitioning (karyokinesis). It is a relatively short catamenia of the cell cycle. M phase is complex and highly regulated. The sequence of events is divided into phases, respective to the completion of one set of activities and the showtime of the adjacent. These phases are sequentially known as:
- prophase
- prometaphase
- metaphase
- anaphase
- telophase
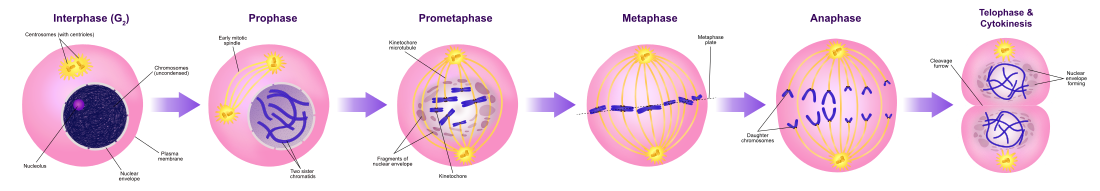
Mitosis is the procedure by which a eukaryotic cell separates the chromosomes in its prison cell nucleus into two identical sets in 2 nuclei.[7] During the process of mitosis the pairs of chromosomes condense and attach to microtubules that pull the sister chromatids to opposite sides of the cell.[viii]
Mitosis occurs exclusively in eukaryotic cells, but occurs in dissimilar ways in dissimilar species. For example, animal cells undergo an "open" mitosis, where the nuclear envelope breaks downwardly before the chromosomes separate, while fungi such as Aspergillus nidulans and Saccharomyces cerevisiae (yeast) undergo a "airtight" mitosis, where chromosomes divide within an intact jail cell nucleus.[9]
Cytokinesis phase (separation of all jail cell components) [edit]
Mitosis is immediately followed by cytokinesis, which divides the nuclei, cytoplasm, organelles and cell membrane into two cells containing roughly equal shares of these cellular components. Mitosis and cytokinesis together define the division of the mother jail cell into ii daughter cells, genetically identical to each other and to their parent prison cell. This accounts for approximately ten% of the prison cell cycle.
Considering cytokinesis usually occurs in conjunction with mitosis, "mitosis" is often used interchangeably with "Yard phase". However, at that place are many cells where mitosis and cytokinesis occur separately, forming unmarried cells with multiple nuclei in a process called endoreplication. This occurs about notably amidst the fungi and slime molds, but is found in various groups. Even in animals, cytokinesis and mitosis may occur independently, for instance during certain stages of fruit fly embryonic development.[10] Errors in mitosis can outcome in cell death through apoptosis or cause mutations that may atomic number 82 to cancer.
Regulation of eukaryotic prison cell bike [edit]

Levels of the three major cyclin types oscillate during the cell cycle (top), providing the basis for oscillations in the cyclin–Cdk complexes that drive jail cell-cycle events (lesser). In general, Cdk levels are constant and in big excess over cyclin levels; thus, cyclin–Cdk complexes course in parallel with cyclin levels. The enzymatic activities of cyclin–Cdk complexes also tend to rising and fall in parallel with cyclin levels, although in some cases Cdk inhibitor proteins or phosphorylation introduce a filibuster between the formation and activation of cyclin–Cdk complexes. Formation of active G1/Southward–Cdk complexes commits the jail cell to a new division bike at the Commencement checkpoint in late G1. G1/Southward–Cdks then activate the Southward–Cdk complexes that initiate Deoxyribonucleic acid replication at the commencement of S phase. G–Cdk activation occurs after the completion of S stage, resulting in progression through the G2/One thousand checkpoint and assembly of the mitotic spindle. APC activation and then triggers sister-chromatid separation at the metaphase-to-anaphase transition. APC activity also causes the destruction of Southward and Thousand cyclins and thus the inactivation of Cdks, which promotes the completion of mitosis and cytokinesis. APC activity is maintained in G1 until G1/S–Cdk activity rises once again and commits the prison cell to the next cycle. This scheme serves only as a full general guide and does not utilise to all cell types.
Regulation of the cell cycle involves processes crucial to the survival of a cell, including the detection and repair of genetic damage besides every bit the prevention of uncontrolled cell partition. The molecular events that control the cell cycle are ordered and directional; that is, each process occurs in a sequential fashion and it is impossible to "reverse" the cycle.
Function of cyclins and CDKs [edit]
Ii fundamental classes of regulatory molecules, cyclins and cyclin-dependent kinases (CDKs), decide a jail cell'south progress through the cell bicycle.[11] Leland H. Hartwell, R. Timothy Chase, and Paul Yard. Nurse won the 2001 Nobel Prize in Physiology or Medicine for their discovery of these primal molecules.[12] Many of the genes encoding cyclins and CDKs are conserved among all eukaryotes, just in general, more circuitous organisms have more than elaborate prison cell bike control systems that comprise more individual components. Many of the relevant genes were starting time identified by studying yeast, especially Saccharomyces cerevisiae;[13] genetic nomenclature in yeast dubs many of these genes cdc (for "cell partition cycle") followed by an identifying number, e.thousand. cdc25 or cdc20.
Cyclins form the regulatory subunits and CDKs the catalytic subunits of an activated heterodimer; cyclins take no catalytic activeness and CDKs are inactive in the absenteeism of a partner cyclin. When activated by a spring cyclin, CDKs perform a mutual biochemical reaction called phosphorylation that activates or inactivates target proteins to orchestrate coordinated entry into the next phase of the jail cell cycle. Different cyclin-CDK combinations determine the downstream proteins targeted. CDKs are constitutively expressed in cells whereas cyclins are synthesised at specific stages of the cell wheel, in response to diverse molecular signals.[14]
General machinery of cyclin-CDK interaction [edit]
Upon receiving a pro-mitotic extracellular signal, Chiliad1 cyclin-CDK complexes become active to prepare the jail cell for S stage, promoting the expression of transcription factors that in turn promote the expression of Southward cyclins and of enzymes required for Dna replication. The Chiliadone cyclin-CDK complexes too promote the degradation of molecules that role as South phase inhibitors by targeting them for ubiquitination. One time a protein has been ubiquitinated, it is targeted for proteolytic degradation by the proteasome. Even so, results from a recent study of E2F transcriptional dynamics at the single-prison cell level argue that the role of G1 cyclin-CDK activities, in particular cyclin D-CDK4/vi, is to tune the timing rather than the commitment of prison cell cycle entry.[15]
Active S cyclin-CDK complexes phosphorylate proteins that make up the pre-replication complexes assembled during G1 phase on Deoxyribonucleic acid replication origins. The phosphorylation serves ii purposes: to activate each already-assembled pre-replication circuitous, and to prevent new complexes from forming. This ensures that every portion of the cell's genome volition be replicated once and simply once. The reason for prevention of gaps in replication is fairly clear, considering daughter cells that are missing all or function of crucial genes will die. However, for reasons related to cistron copy number effects, possession of extra copies of sure genes is too deleterious to the girl cells.
Mitotic cyclin-CDK complexes, which are synthesized but inactivated during S and K2 phases, promote the initiation of mitosis by stimulating downstream proteins involved in chromosome condensation and mitotic spindle assembly. A critical complex activated during this process is a ubiquitin ligase known as the anaphase-promoting circuitous (APC), which promotes degradation of structural proteins associated with the chromosomal kinetochore. APC too targets the mitotic cyclins for deposition, ensuring that telophase and cytokinesis tin can proceed.[16]
Specific activeness of cyclin-CDK complexes [edit]
Cyclin D is the get-go cyclin produced in the cells that enter the prison cell cycle, in response to extracellular signals (e.g. growth factors). Cyclin D levels stay low in resting cells that are not proliferating. Additionally, CDK4/6 and CDK2 are also inactive because CDK4/vi are jump past INK4 family members (eastward.g., p16), limiting kinase activity. Meanwhile, CDK2 complexes are inhibited by the CIP/KIP proteins such as p21 and p27,[17] When it is fourth dimension for a cell to enter the jail cell cycle, which is triggered by a mitogenic stimuli, levels of cyclin D increment. In response to this trigger, cyclin D binds to existing CDK4/6, forming the active cyclin D-CDK4/half-dozen complex. Cyclin D-CDK4/6 complexes in turn mono-phosphorylates the retinoblastoma susceptibility protein (Rb) to pRb. The united nations-phosphorylated Rb tumour suppressor functions in inducing cell cycle leave and maintaining G0 arrest (senescence).[18]
In the last few decades, a model has been widely accustomed whereby pRB proteins are inactivated by cyclin D-Cdk4/6-mediated phosphorylation. Rb has 14+ potential phosphorylation sites. Cyclin D-Cdk four/6 progressively phosphorylates Rb to hyperphosphorylated state, which triggers dissociation of pRB–E2F complexes, thereby inducing G1/S cell wheel gene expression and progression into S stage.[nineteen]
However, scientific observations from a contempo written report show that Rb is present in three types of isoforms: (ane) united nations-phosphorylated Rb in G0 state; (ii) mono-phosphorylated Rb, as well referred to equally "hypo-phosphorylated' or 'partially' phosphorylated Rb in early on G1 state; and (3) inactive hyper-phosphorylated Rb in late G1 land.[20] [21] [22] In early G1 cells, mono-phosphorylated Rb exits equally 14 unlike isoforms, ane of each has distinct E2F bounden affinity.[22] Rb has been plant to associate with hundreds of different proteins[23] and the idea that dissimilar mono-phosphorylated Rb isoforms have dissimilar protein partners was very appealing.[24] A contempo study confirmed that mono-phosphorylation controls Rb's clan with other proteins and generates functional distinct forms of Rb.[25] All different mono-phosphorylated Rb isoforms inhibit E2F transcriptional program and are able to arrest cells in G1-phase. Importantly, unlike mono-phosphorylated forms of RB have distinct transcriptional outputs that are extended beyond E2F regulation.[25]
In general, the binding of pRb to E2F inhibits the E2F target gene expression of certain G1/Due south and S transition genes including E-type cyclins. The partial phosphorylation of RB de-represses the Rb-mediated suppression of E2F target factor expression, begins the expression of cyclin E. The molecular mechanism that causes the cell switched to cyclin Due east activation is currently not known, but as cyclin E levels rise, the agile cyclin Eastward-CDK2 complex is formed, bringing Rb to be inactivated by hyper-phosphorylation.[22] Hyperphosphorylated Rb is completely dissociated from E2F, enabling further expression of a wide range of E2F target genes are required for driving cells to proceed into Due south phase [1]. Recently, it has been identified that cyclin D-Cdk4/6 binds to a C-concluding blastoff-helix region of Rb that is just distinguishable to cyclin D rather than other cyclins, cyclin Eastward, A and B.[26] This ascertainment based on the structural analysis of Rb phosphorylation supports that Rb is phosphorylated in a different level through multiple Cyclin-Cdk complexes. This also makes viable the current model of a simultaneous switch-like inactivation of all mono-phosphorylated Rb isoforms through one type of Rb hyper-phosphorylation mechanism. In add-on, mutational analysis of the cyclin D- Cdk 4/6 specific Rb C-final helix shows that disruptions of cyclin D-Cdk 4/six bounden to Rb prevents Rb phosphorylation, arrests cells in G1, and bolsters Rb'due south functions in tumor suppressor.[26] This cyclin-Cdk driven jail cell cycle transitional mechanism governs a cell committed to the cell cycle that allows cell proliferation. A cancerous jail cell growth often accompanies with deregulation of Cyclin D-Cdk 4/6 activity.
The hyperphosphorylated Rb dissociates from the E2F/DP1/Rb complex (which was spring to the E2F responsive genes, effectively "blocking" them from transcription), activating E2F. Activation of E2F results in transcription of various genes similar cyclin East, cyclin A, Dna polymerase, thymidine kinase, etc. Cyclin E thus produced binds to CDK2, forming the cyclin Eastward-CDK2 complex, which pushes the prison cell from Thousand1 to S phase (G1/S, which initiates the Kii/M transition).[27] Cyclin B-cdk1 complex activation causes breakup of nuclear envelope and initiation of prophase, and subsequently, its deactivation causes the prison cell to exit mitosis.[14] A quantitative study of E2F transcriptional dynamics at the single-cell level by using engineered fluorescent reporter cells provided a quantitative framework for understanding the control logic of jail cell cycle entry, challenging the canonical textbook model. Genes that regulate the amplitude of E2F accumulation, such equally Myc, determine the delivery in cell bicycle and S phase entry. G1 cyclin-CDK activities are not the driver of prison cell wheel entry. Instead, they primarily tune the timing of E2F increase, thereby modulating the pace of cell cycle progression.[xv]
Inhibitors [edit]
Endogenous [edit]
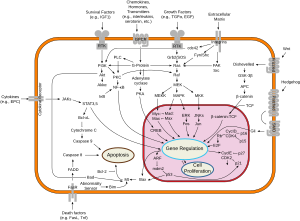
Overview of point transduction pathways involved in apoptosis, also known every bit "programmed cell death"
Two families of genes, the cip/kip (CDK interacting protein/Kinase inhibitory protein) family and the INK4a/ARF (Inhibitor of 1000inase 4/Alternative Reading Frame) family unit, prevent the progression of the jail cell cycle. Because these genes are instrumental in prevention of tumor germination, they are known as tumor suppressors.
The cip/kip family includes the genes p21, p27 and p57. They halt the cell bicycle in Chiliadane phase by binding to and inactivating cyclin-CDK complexes. p21 is activated by p53 (which, in turn, is triggered past DNA damage due east.g. due to radiations). p27 is activated past Transforming Growth Gene β (TGF β), a growth inhibitor.
The INK4a/ARF family unit includes p16INK4a, which binds to CDK4 and arrests the cell bicycle in M1 phase, and p14ARF which prevents p53 degradation.
Synthetic [edit]
Synthetic inhibitors of Cdc25 could also be useful for the arrest of cell cycle and therefore be useful as antineoplastic and anticancer agents.[28]
Many homo cancers possess the hyper-activated Cdk four/vi activities.[29] Given the observations of cyclin D-Cdk four/6 functions, inhibition of Cdk 4/6 should result in preventing a cancerous tumor from proliferating. Consequently, scientists have tried to invent the constructed Cdk4/6 inhibitor as Cdk4/half dozen has been characterized to exist a therapeutic target for anti-tumor effectiveness. Three Cdk4/half dozen inhibitors - palbociclib, ribociclib, and abemaciclib - currently received FDA approval for clinical use to treat advanced-stage or metastatic, hormone-receptor-positive (HR-positive, HR+), HER2-negative (HER2-) breast cancer.[xxx] [31] For example, palbociclib is an orally active CDK4/vi inhibitor which has demonstrated improved outcomes for ER-positive/HER2-negative advanced breast cancer. The main side consequence is neutropenia which can be managed past dose reduction.[32]
Cdk4/6 targeted therapy will only treat cancer types where Rb is expressed. Cancer cells with loss of Rb have principal resistance to Cdk4/six inhibitors.
Transcriptional regulatory network [edit]
Current prove suggests that a semi-democratic transcriptional network acts in concert with the CDK-cyclin machinery to regulate the prison cell cycle. Several factor expression studies in Saccharomyces cerevisiae have identified 800–1200 genes that modify expression over the course of the cell cycle.[13] [33] [34] They are transcribed at loftier levels at specific points in the cell bicycle, and remain at lower levels throughout the rest of the cycle. While the gear up of identified genes differs between studies due to the computational methods and criteria used to identify them, each report indicates that a big portion of yeast genes are temporally regulated.[35]
Many periodically expressed genes are driven by transcription factors that are besides periodically expressed. One screen of single-gene knockouts identified 48 transcription factors (nigh 20% of all non-essential transcription factors) that prove cell cycle progression defects.[36] Genome-broad studies using high throughput technologies accept identified the transcription factors that demark to the promoters of yeast genes, and correlating these findings with temporal expression patterns take allowed the identification of transcription factors that drive stage-specific cistron expression.[33] [37] The expression profiles of these transcription factors are driven by the transcription factors that summit in the prior stage, and computational models take shown that a CDK-democratic network of these transcription factors is sufficient to produce steady-state oscillations in gene expression).[34] [38]
Experimental evidence as well suggests that gene expression tin can oscillate with the period seen in dividing wild-type cells independently of the CDK machinery. Orlando et al. used microarrays to measure out the expression of a ready of 1,271 genes that they identified as periodic in both wild type cells and cells lacking all Due south-stage and mitotic cyclins (clb1,2,iii,4,five,6). Of the 1,271 genes assayed, 882 continued to exist expressed in the cyclin-deficient cells at the aforementioned fourth dimension every bit in the wild type cells, despite the fact that the cyclin-deficient cells abort at the edge between Thousandi and S phase. Nevertheless, 833 of the genes assayed changed behavior between the wild type and mutant cells, indicating that these genes are likely straight or indirectly regulated by the CDK-cyclin machinery. Some genes that continued to be expressed on fourth dimension in the mutant cells were also expressed at unlike levels in the mutant and wild type cells. These findings suggest that while the transcriptional network may oscillate independently of the CDK-cyclin oscillator, they are coupled in a manner that requires both to ensure the proper timing of jail cell bike events.[34] Other work indicates that phosphorylation, a post-translational modification, of cell bike transcription factors by Cdk1 may alter the localization or activity of the transcription factors in guild to tightly control timing of target genes.[36] [39] [40]
While oscillatory transcription plays a key role in the progression of the yeast cell wheel, the CDK-cyclin machinery operates independently in the early embryonic cell cycle. Earlier the midblastula transition, zygotic transcription does non occur and all needed proteins, such as the B-type cyclins, are translated from maternally loaded mRNA.[41]
Deoxyribonucleic acid replication and DNA replication origin activeness [edit]
Analyses of synchronized cultures of Saccharomyces cerevisiae nether conditions that foreclose DNA replication initiation without delaying cell cycle progression showed that origin licensing decreases the expression of genes with origins near their 3' ends, revealing that downstream origins can regulate the expression of upstream genes.[42] This confirms previous predictions from mathematical modeling of a global causal coordination betwixt Dna replication origin activity and mRNA expression,[43] [44] [45] and shows that mathematical modeling of DNA microarray data tin be used to correctly predict previously unknown biological modes of regulation.
Checkpoints [edit]
Cell bicycle checkpoints are used by the cell to monitor and regulate the progress of the cell bicycle.[46] Checkpoints prevent jail cell bicycle progression at specific points, assuasive verification of necessary phase processes and repair of DNA harm. The cell cannot go on to the next phase until checkpoint requirements accept been met. Checkpoints typically consist of a network of regulatory proteins that monitor and dictate the progression of the jail cell through the different stages of the jail cell cycle.
It is estimated that in normal human cells about 1% of unmarried-strand DNA damages are converted to about l endogenous DNA double-strand breaks per jail cell per jail cell bike.[47] Although such double-strand breaks are usually repaired with high fidelity, errors in their repair are considered to contribute significantly to the rate of cancer in humans.[47]
There are several checkpoints to ensure that damaged or incomplete Deoxyribonucleic acid is non passed on to daughter cells. 3 main checkpoints exist: the Grandone/S checkpoint, the Gii/Thousand checkpoint and the metaphase (mitotic) checkpoint. Another checkpoint is the Get checkpoint, in which the cells are checked for maturity. If the cells fail to pass this checkpoint by not being ready withal, they volition be discarded from dividing.
Gane/South transition is a rate-limiting footstep in the prison cell cycle and is also known as restriction point.[14] This is where the cell checks whether information technology has enough raw materials to fully replicate its DNA (nucleotide bases, Dna synthase, chromatin, etc.). An unhealthy or malnourished cell will become stuck at this checkpoint.
The G2/G checkpoint is where the cell ensures that it has enough cytoplasm and phospholipids for 2 daughter cells. But sometimes more than chiefly, it checks to run across if it is the right time to replicate. There are some situations where many cells need to all replicate simultaneously (for example, a growing embryo should have a symmetric cell distribution until it reaches the mid-blastula transition). This is done by controlling the G2/K checkpoint.
The metaphase checkpoint is a fairly minor checkpoint, in that once a cell is in metaphase, information technology has committed to undergoing mitosis. However that's not to say information technology isn't important. In this checkpoint, the jail cell checks to ensure that the spindle has formed and that all of the chromosomes are aligned at the spindle equator before anaphase begins.[48]
While these are the three "master" checkpoints, not all cells have to pass through each of these checkpoints in this order to replicate. Many types of cancer are caused past mutations that allow the cells to speed through the various checkpoints or fifty-fifty skip them altogether. Going from S to Grand to S phase almost consecutively. Because these cells accept lost their checkpoints, any DNA mutations that may have occurred are disregarded and passed on to the daughter cells. This is one reason why cancer cells take a trend to exponentially accumulate mutations. Bated from cancer cells, many fully differentiated prison cell types no longer replicate so they leave the cell bike and stay in G0 until their death. Thus removing the need for cellular checkpoints. An culling model of the cell cycle response to Dna impairment has too been proposed, known as the postreplication checkpoint.
Checkpoint regulation plays an important office in an organism'southward development. In sexual reproduction, when egg fertilization occurs, when the sperm binds to the egg, it releases signalling factors that notify the egg that information technology has been fertilized. Among other things, this induces the now fertilized oocyte to return from its previously dormant, G0, state back into the prison cell wheel and on to mitotic replication and division.
p53 plays an important role in triggering the control mechanisms at both G1/Southward and G2/M checkpoints. In improver to p53, checkpoint regulators are existence heavily researched for their roles in cancer growth and proliferation.
Fluorescence imaging of the jail cell bicycle [edit]

Fluorescent proteins visualize the cell cycle progression. IFP2.0-hGem(1/110) fluorescence is shown in dark-green and highlights the South/K2/M phases. smURFP-hCdtI(30/120) fluorescence is shown in red and highlights the G0/M1 phases.
Pioneering work by Atsushi Miyawaki and coworkers developed the fluorescent ubiquitination-based cell cycle indicator (FUCCI), which enables fluorescence imaging of the cell cycle. Originally, a green fluorescent protein, magazine, was fused to hGem(1/110) and an orangish fluorescent protein (mKO2) was fused to hCdt1(30/120). Note, these fusions are fragments that comprise a nuclear localization point and ubiquitination sites for degradation, but are non functional proteins. The dark-green fluorescent protein is made during the S, G2, or M phase and degraded during the One thousand0 or Thousand1 stage, while the orange fluorescent protein is fabricated during the Grand0 or G1 phase and destroyed during the South, G2, or G phase.[49] A far-red and near-infrared FUCCI was developed using a blue-green alga-derived fluorescent protein (smURFP) and a bacteriophytochrome-derived fluorescent protein (movie institute at this link).[fifty]
Role in tumor formation [edit]
A disregulation of the cell cycle components may lead to tumor formation.[51] Equally mentioned above, when some genes like the cell cycle inhibitors, RB, p53 etc. mutate, they may cause the prison cell to multiply uncontrollably, forming a tumor. Although the duration of cell bicycle in tumor cells is equal to or longer than that of normal cell cycle, the proportion of cells that are in active cell division (versus quiescent cells in 10000 phase) in tumors is much higher than that in normal tissue.[52] Thus at that place is a net increase in jail cell number as the number of cells that die by apoptosis or senescence remains the same.
The cells which are actively undergoing cell cycle are targeted in cancer therapy as the DNA is relatively exposed during jail cell sectionalization and hence susceptible to harm by drugs or radiation. This fact is made use of in cancer treatment; by a process known as debulking, a significant mass of the tumor is removed which pushes a significant number of the remaining tumor cells from G0 to Gane phase (due to increased availability of nutrients, oxygen, growth factors etc.). Radiation or chemotherapy following the debulking procedure kills these cells which accept newly entered the cell wheel.[14]
The fastest cycling mammalian cells in culture, crypt cells in the intestinal epithelium, have a cycle fourth dimension as curt as 9 to 10 hours. Stalk cells in resting mouse skin may take a cycle fourth dimension of more than than 200 hours. Nearly of this difference is due to the varying length of K1, the most variable phase of the cycle. M and S do not vary much.
In general, cells are most radiosensitive in late Yard and Thouii phases and most resistant in late S phase.
For cells with a longer cell cycle fourth dimension and a significantly long G1 phase, there is a second peak of resistance late in G1.
The pattern of resistance and sensitivity correlates with the level of sulfhydryl compounds in the cell. Sulfhydryls are natural substances that protect cells from radiations damage and tend to be at their highest levels in S and at their everyman near mitosis.
Homologous recombination (HR) is an accurate process for repairing DNA double-strand breaks. 60 minutes is nearly absent in G1 phase, is most active in S phase, and declines in M2/Grand.[53] Not-homologous end joining, a less authentic and more mutagenic process for repairing double strand breaks, is agile throughout the cell cycle.
Run across also [edit]
- Cellular model
- Eukaryotic Deoxyribonucleic acid replication
- Origin recognition complex
- Retinoblastoma poly peptide
- Synchronous culture – synchronization of cell cultures
- Wee1
References [edit]
- ^ Wang JD, Levin PA (November 2009). "Metabolism, cell growth and the bacterial cell wheel". Nature Reviews. Microbiology. 7 (11): 822–7. doi:10.1038/nrmicro2202. PMC2887316. PMID 19806155.
- ^ Cooper GM (2000). "Chapter 14: The Eukaryotic Jail cell Cycle". The cell: a molecular approach (2d ed.). Washington, D.C: ASM Press. ISBN978-0-87893-106-iv.
- ^ Smith JA, Martin L (April 1973). "Do cells cycle?". Proceedings of the National Academy of Sciences of the United States of America. seventy (4): 1263–7. Bibcode:1973PNAS...70.1263S. doi:10.1073/pnas.70.four.1263. PMC433472. PMID 4515625.
- ^ Wu RS, Bonner WM (December 1981). "Separation of basal histone synthesis from Due south-stage histone synthesis in dividing cells". Cell. 27 (2 Pt 1): 321–30. doi:10.1016/0092-8674(81)90415-3. PMID 7199388. S2CID 12215040.
- ^ Nelson DM, Ye X, Hall C, Santos H, Ma T, Kao GD, et al. (November 2002). "Coupling of DNA synthesis and histone synthesis in Due south phase independent of cyclin/cdk2 action". Molecular and Cellular Biology. 22 (21): 7459–72. doi:10.1128/MCB.22.21.7459-7472.2002. PMC135676. PMID 12370293.
- ^ Cameron IL, Greulich RC (July 1963). "Evidence for an essentially constant duration of DNA synthesis in renewing epithelia of the adult mouse". The Journal of Prison cell Biological science. xviii: 31–40. doi:ten.1083/jcb.18.1.31. PMC2106275. PMID 14018040.
- ^ Rubenstein I, Wick SM (2008). "Cell". World Book Online Reference Center. Archived from the original on 30 May 2011. Retrieved 10 July 2009.
- ^ Maton A, Lahart D, Hopkins J, Warner MQ, Johnson S, Wright JD (1997). Cells: Building Blocks of Life. New Jersey: Prentice Hall. pp. 70–4. ISBN978-0-13-423476-two.
- ^ De Souza CP, Osmani SA (September 2007). "Mitosis, not just open or closed". Eukaryotic Jail cell. 6 (9): 1521–7. doi:10.1128/EC.00178-07. PMC2043359. PMID 17660363.
- ^ Lilly MA, Duronio RJ (Apr 2005). "New insights into cell bike command from the Drosophila endocycle". Oncogene. 24 (17): 2765–75. doi:x.1038/sj.onc.1208610. PMID 15838513.
- ^ Nigg EA (June 1995). "Cyclin-dependent poly peptide kinases: central regulators of the eukaryotic prison cell cycle". BioEssays. 17 (6): 471–80. doi:ten.1002/bies.950170603. PMID 7575488. S2CID 44307473.
- ^ "The Nobel Prize in Physiology or Medicine 2001 - Press release". Nobelprize.org.
- ^ a b Spellman PT, Sherlock Thousand, Zhang MQ, Iyer VR, Anders K, Eisen MB, et al. (December 1998). "Comprehensive identification of cell bicycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization". Molecular Biological science of the Jail cell. 9 (12): 3273–97. doi:x.1091/mbc.9.12.3273. PMC25624. PMID 9843569.
- ^ a b c d Robbins SL, Cotran RS (2004). Kumar 5, Abbas AK, Fausto North (eds.). Pathological Basis of Illness. Elsevier. ISBN978-81-8147-528-ii.
- ^ a b Dong P, Maddali MV, Srimani JK, Thélot F, Nevins JR, Mathey-Prevot B, You 50 (September 2014). "Sectionalisation of labour between Myc and G1 cyclins in jail cell cycle commitment and pace control". Nature Communications. v: 4750. Bibcode:2014NatCo...5.4750D. doi:10.1038/ncomms5750. PMC4164785. PMID 25175461.
- ^ Mahmoudi M, Azadmanesh 1000, Shokrgozar MA, Journeay WS, Laurent S (May 2011). "Effect of nanoparticles on the prison cell life wheel". Chemical Reviews. 111 (5): 3407–32. doi:10.1021/cr1003166. PMID 21401073.
- ^ Goel S, DeCristo MJ, McAllister SS, Zhao JJ (November 2018). "CDK4/vi Inhibition in Cancer: Across Prison cell Bike Arrest". Trends in Jail cell Biology. 28 (11): 911–925. doi:10.1016/j.tcb.2018.07.002. PMC6689321. PMID 30061045.
- ^ Burkhart DL, Sage J (September 2008). "Cellular mechanisms of tumour suppression by the retinoblastoma gene". Nature Reviews. Cancer. 8 (9): 671–82. doi:x.1038/nrc2399. PMC6996492. PMID 18650841.
- ^ Morgan DO (2007). The cell cycle : principles of command. London: New Science Press. ISBN978-0-19-920610-0. OCLC 70173205.
- ^ Paternot S, Bockstaele 50, Bisteau 10, Kooken H, Coulonval K, Roger PP (February 2010). "Rb inactivation in prison cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase". Cell Cycle. nine (four): 689–99. doi:10.4161/cc.9.4.10611. PMID 20107323.
- ^ Henley SA, Dick FA (March 2012). "The retinoblastoma family of proteins and their regulatory functions in the mammalian cell partitioning cycle". Cell Sectionalization. 7 (1): 10. doi:10.1186/1747-1028-7-10. PMC3325851. PMID 22417103.
- ^ a b c Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF (June 2014). "Cyclin D activates the Rb tumor suppressor past mono-phosphorylation". eLife. 3: e02872. doi:ten.7554/eLife.02872. PMC4076869. PMID 24876129.
- ^ Morris EJ, Dyson NJ (1 Jan 2001). Retinoblastoma poly peptide partners. Advances in Cancer Research. Vol. 82. Academic Press. pp. 1–54. doi:10.1016/s0065-230x(01)82001-7. ISBN9780120066827. PMID 11447760.
- ^ Dyson NJ (July 2016). "RB1: a prototype tumor suppressor and an enigma". Genes & Development. 30 (xiii): 1492–502. doi:10.1101/gad.282145.116. PMC4949322. PMID 27401552.
- ^ a b Sanidas I, Morris R, Fella KA, Rumde PH, Boukhali M, Tai EC, et al. (March 2019). "A Code of Mono-phosphorylation Modulates the Function of RB". Molecular Prison cell. 73 (5): 985–thou.e6. doi:ten.1016/j.molcel.2019.01.004. PMC6424368. PMID 30711375.
- ^ a b Topacio BR, Zatulovskiy E, Cristea Due south, Xie S, Tambo CS, Rubin SM, et al. (May 2019). "Cyclin D-Cdk4,vi Drives Cell-Cycle Progression via the Retinoblastoma Protein'southward C-Terminal Helix". Molecular Cell. 74 (4): 758–770.e4. doi:10.1016/j.molcel.2019.03.020. PMC6800134. PMID 30982746.
- ^ Norbury C (1995). "Cdk2 protein kinase (vertebrates)". In Hardie DG, Hanks S (eds.). Poly peptide kinase factsBook. Boston: Bookish Printing. pp. 184. ISBN978-0-12-324719-3.
- ^ "Presentation on CDC25 PHOSPHATASES: A Potential Target for Novel Anticancer Agents". Archived from the original on 3 March 2016. Retrieved 11 March 2010.
- ^ Sherr CJ, Beach D, Shapiro GI (April 2016). "Targeting CDK4 and CDK6: From Discovery to Therapy". Cancer Discovery. 6 (four): 353–67. doi:10.1158/2159-8290.cd-15-0894. PMC4821753. PMID 26658964.
- ^ O'Leary B, Finn RS, Turner NC (July 2016). "Treating cancer with selective CDK4/6 inhibitors". Nature Reviews. Clinical Oncology. thirteen (seven): 417–xxx. doi:ten.1038/nrclinonc.2016.26. PMID 27030077. S2CID 23646632.
- ^ Bilgin B, Sendur MA, Şener Dede D, Akıncı MB, Yalçın B (September 2017). "A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer". Current Medical Research and Opinion. 33 (9): 1559–1569. doi:10.1080/03007995.2017.1348344. PMID 28657360. S2CID 205542255.
- ^ Schmidt M, Sebastian M (August 2018). "Palbociclib-The Beginning of a New Class of Prison cell Cycle Inhibitors". Recent Results in Cancer Research. Fortschritte der Krebsforschung. Progres dans les Recherches Sur le Cancer. Recent Results in Cancer Research. 211: 153–175. doi:ten.1007/978-three-319-91442-8_11. ISBN978-three-319-91441-1. PMID 30069766.
- ^ a b Pramila T, Wu W, Miles S, Noble WS, Breeden LL (Baronial 2006). "The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the jail cell wheel". Genes & Evolution. 20 (16): 2266–78. doi:10.1101/gad.1450606. PMC1553209. PMID 16912276.
- ^ a b c Orlando DA, Lin CY, Bernard A, Wang JY, Socolar JE, Iversen ES, et al. (June 2008). "Global control of prison cell-bicycle transcription past coupled CDK and network oscillators". Nature. 453 (7197): 944–vii. Bibcode:2008Natur.453..944O. doi:10.1038/nature06955. PMC2736871. PMID 18463633.
- ^ de Lichtenberg U, Jensen LJ, Fausbøll A, Jensen TS, Bork P, Brunak S (April 2005). "Comparing of computational methods for the identification of jail cell cycle-regulated genes". Bioinformatics. 21 (vii): 1164–71. doi:10.1093/bioinformatics/bti093. PMID 15513999.
- ^ a b White MA, Riles L, Cohen BA (February 2009). "A systematic screen for transcriptional regulators of the yeast cell cycle". Genetics. 181 (2): 435–46. doi:10.1534/genetics.108.098145. PMC2644938. PMID 19033152.
- ^ Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, et al. (October 2002). "Transcriptional regulatory networks in Saccharomyces cerevisiae". Science. 298 (5594): 799–804. Bibcode:2002Sci...298..799L. doi:10.1126/science.1075090. PMID 12399584. S2CID 4841222.
- ^ Simon I, Barnett J, Hannett N, Harbison CT, Rinaldi NJ, Volkert TL, et al. (September 2001). "Serial regulation of transcriptional regulators in the yeast jail cell cycle". Cell. 106 (vi): 697–708. doi:10.1016/S0092-8674(01)00494-9. PMID 11572776. S2CID 9308235.
- ^ Sidorova JM, Mikesell GE, Breeden LL (Dec 1995). "Cell cycle-regulated phosphorylation of Swi6 controls its nuclear localization". Molecular Biology of the Cell. 6 (12): 1641–58. doi:ten.1091/mbc.6.12.1641. PMC301322. PMID 8590795.
- ^ Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah M, et al. (October 2003). "Targets of the cyclin-dependent kinase Cdk1". Nature. 425 (6960): 859–64. Bibcode:2003Natur.425..859U. doi:10.1038/nature02062. PMID 14574415. S2CID 4391711.
- ^ Morgan DO (2007). "2–3". The Cell Cycle: Principles of Control. London: New Scientific discipline Press. p. 18. ISBN978-0-9539181-2-vi.
- ^ Omberg Fifty, Meyerson JR, Kobayashi Chiliad, Drury LS, Diffley JF, Alter O (October 2009). "Global effects of DNA replication and Dna replication origin activity on eukaryotic gene expression". Molecular Systems Biological science. 5: 312. doi:10.1038/msb.2009.lxx. PMC2779084. PMID 19888207.
- ^ Modify O, Golub GH, Brownish PO, Botstein D (February 2004). Deutscher MP, Black S, Boehmer PE, D'Urso G, Fletcher TM, Huijing F, et al. (eds.). Novel Genome-Scale Correlation between Dna Replication and RNA Transcription During the Prison cell Bicycle in Yeast is Predicted past Data-Driven Models (PDF). Miami Nature Biotechnology Wintertime Symposium. Prison cell Cycle, Chromosomes and Cancer. Vol. 15. Miami Embankment, FL: University of Miami School of Medicine.
- ^ Alter O, Golub GH (November 2004). "Integrative analysis of genome-scale information by using pseudoinverse projection predicts novel correlation between Dna replication and RNA transcription". Proceedings of the National Academy of Sciences of the United states of america of America. 101 (47): 16577–82. Bibcode:2004PNAS..10116577A. doi:x.1073/pnas.0406767101. PMC534520. PMID 15545604.
- ^ Omberg Fifty, Golub GH, Alter O (November 2007). "A tensor higher-order singular value decomposition for integrative analysis of Dna microarray information from different studies". Proceedings of the National University of Sciences of the Usa of America. 104 (47): 18371–six. Bibcode:2007PNAS..10418371O. doi:ten.1073/pnas.0709146104. PMC2147680. PMID 18003902.
- ^ Elledge SJ (Dec 1996). "Cell cycle checkpoints: preventing an identity crunch". Science. 274 (5293): 1664–72. Bibcode:1996Sci...274.1664E. doi:10.1126/science.274.5293.1664. PMID 8939848. S2CID 39235426.
- ^ a b Vilenchik MM, Knudson AG (October 2003). "Endogenous DNA double-strand breaks: product, fidelity of repair, and induction of cancer". Proceedings of the National Academy of Sciences of the The states of America. 100 (22): 12871–6. doi:10.1073/pnas.2135498100. PMC240711. PMID 14566050.
- ^ LeMaire-Adkins R, Radke K, Hunt PA (December 1997). "Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females". The Journal of Cell Biological science. 139 (seven): 1611–nine. doi:x.1083/jcb.139.7.1611. PMC2132649. PMID 9412457.
- ^ Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. (Feb 2008). "Visualizing spatiotemporal dynamics of multicellular cell-cycle progression". Cell. 132 (3): 487–98. doi:10.1016/j.prison cell.2007.12.033. PMID 18267078. S2CID 15704902.
- ^ Rodriguez EA, Tran GN, Gross LA, Crisp JL, Shu X, Lin JY, Tsien RY (September 2016). "A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein". Nature Methods. xiii (nine): 763–9. doi:ten.1038/nmeth.3935. PMC5007177. PMID 27479328.
- ^ Champeris Tsaniras S, Kanellakis N, Symeonidou IE, Nikolopoulou P, Lygerou Z, Taraviras S (June 2014). "Licensing of Deoxyribonucleic acid replication, cancer, pluripotency and differentiation: an interlinked earth?". Seminars in Prison cell & Developmental Biology. 30: 174–80. doi:10.1016/j.semcdb.2014.03.013. PMID 24641889.
- ^ Baserga R (June 1965). "The Relationship of the Cell Cycle to Tumor Growth and Control of Cell Sectionalisation". Cancer Inquiry. 25 (v): 581–95. PMID 14347544.
- ^ Mao Z, Bozzella K, Seluanov A, Gorbunova 5 (September 2008). "Deoxyribonucleic acid repair by nonhomologous end joining and homologous recombination during cell bicycle in human cells". Cell Bike. 7 (18): 2902–vi. doi:10.4161/cc.7.18.6679. PMC2754209. PMID 18769152.
Farther reading [edit]
- Morgan DO (2007). The Jail cell Cycle: Principles of Command. London: Published past New Science Press in association with Oxford University Press. ISBN978-0-87893-508-vi.
- Alberts B, Johnson A, Lewis J, Raff One thousand, Roberts K, Walter P (2008). "Affiliate 17". Molecular Biology of the Cell (5th ed.). New York: Garland Scientific discipline. ISBN978-0-8153-4111-vi.
- Krieger Thou, Scott MP, Matsudaira PT, Lodish HF, Darnell JE, Zipursky 50, Kaiser C, Berk A (2004). Molecular cell biological science. New York: Westward.H. Freeman and CO. ISBN978-0-7167-4366-8.
- Watson JD, Bakery TA, Bell SP, Gann A, Levine M, Losick R (2004). "Chapter 7". Molecular biology of the gene (fifth ed.). San Francisco: Pearson/Benjamin Cummings. ISBN978-0-8053-4642-8.
External links [edit]
| | Wikimedia Commons has media related to Prison cell cycle. |
-
 This article incorporates public domain cloth from the NCBI certificate: "Science Primer".
This article incorporates public domain cloth from the NCBI certificate: "Science Primer". - David Morgan'southward Seminar: Decision-making the Jail cell Bicycle
- The prison cell bicycle & Cell expiry
- Transcriptional plan of the prison cell cycle: loftier-resolution timing
- Cell cycle and metabolic cycle regulated transcription in yeast
- Cell Cycle Blitheness 1Lec.com
- Cell Cycle
- Fucci:Using GFP to visualize the cell-cycle
- Science Creative Quarterly's overview of the cell bicycle
- KEGG – Human being Jail cell Cycle
Source: https://en.wikipedia.org/wiki/Cell_cycle
0 Response to "In What Stage Is a Cell That Will Not Divide Again"
Post a Comment